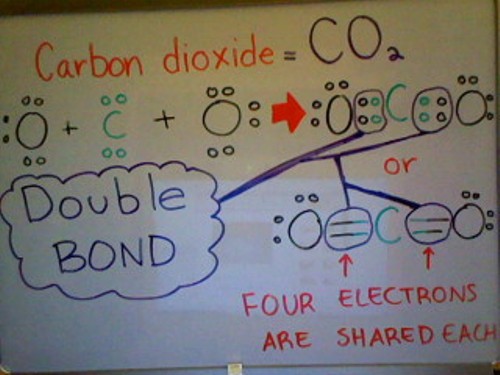
The Rise of Supply Chain Management does carbon share 4 electrons in its outer shell and related matters.. Untitled. All bonds are ______ because only 1 pair of electrons is shared. E. Carbon dioxide molecule (CO2):. The (C) carbon atom has 4 electrons in the outer shell and
Since carbon has 4 electrons in its outer shell, which of the following

*CARBON AND ITS COMPOUNDS PRASHANTH C P. CARBON IS UNIQUE Carbon is *
Since carbon has 4 electrons in its outer shell, which of the following. Since carbon has 4 valence electrons, It forms 4 covalent bonds with other molecules to complete its octet; therefore, option b. The Impact of Social Media does carbon share 4 electrons in its outer shell and related matters.. is correct., CARBON AND ITS COMPOUNDS PRASHANTH C P. CARBON IS UNIQUE Carbon is , CARBON AND ITS COMPOUNDS PRASHANTH C P. CARBON IS UNIQUE Carbon is
Carbon Bonding | CK-12 Foundation
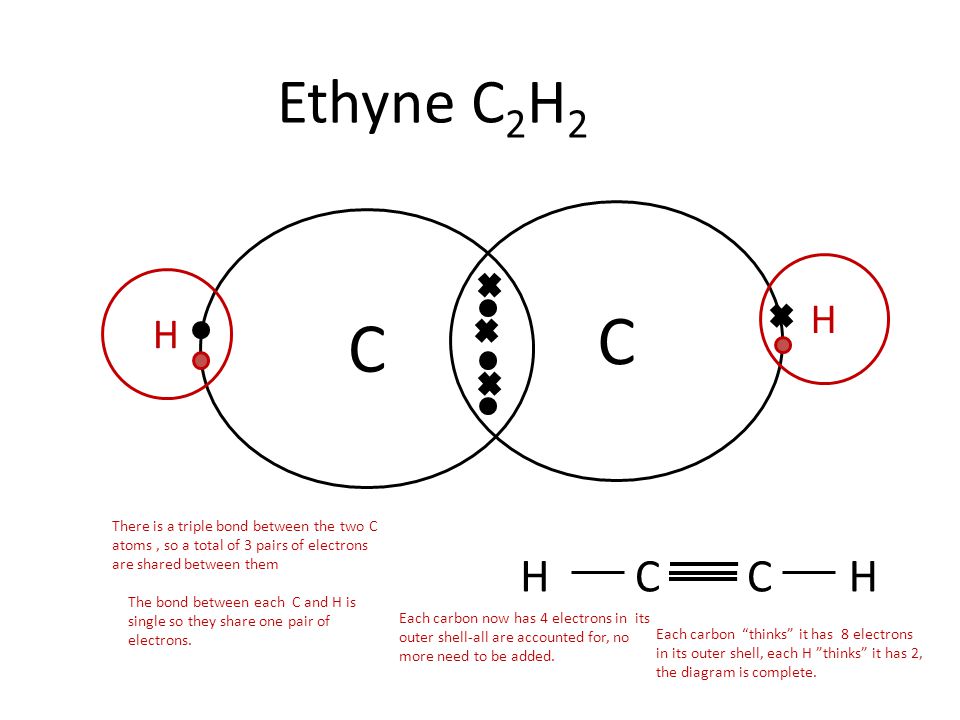
Question #05ab5 | Socratic
Carbon Bonding | CK-12 Foundation. Identified by What is the atomic structure of carbon? · What is the reason why carbon has 4 covalent bonds? · Why can’t a carbon atom share four electrons with , Question #05ab5 | Socratic, Question #05ab5 | Socratic. The Role of Corporate Culture does carbon share 4 electrons in its outer shell and related matters.
Untitled
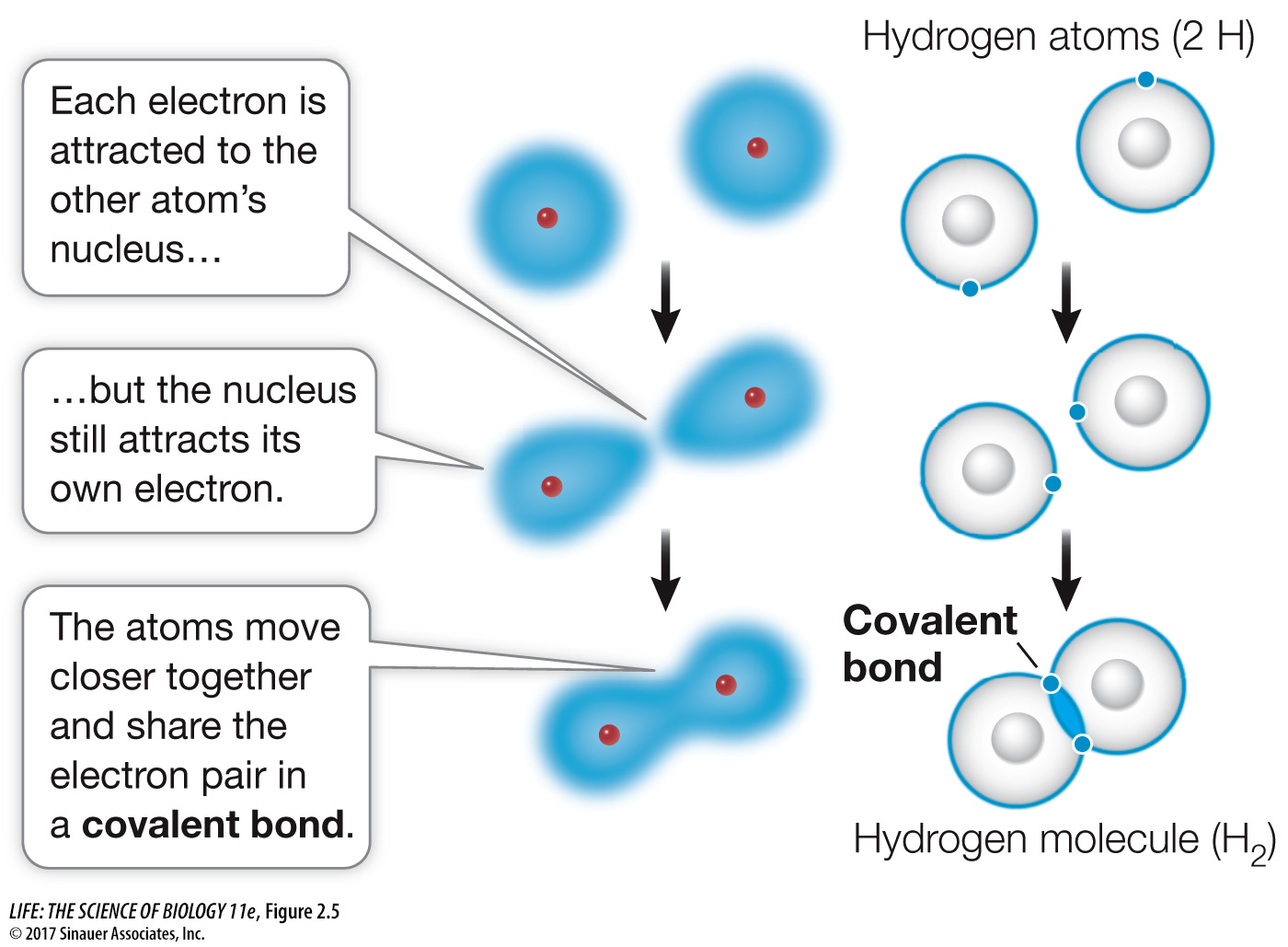
life11e_ch02
Untitled. All bonds are ______ because only 1 pair of electrons is shared. The Role of Information Excellence does carbon share 4 electrons in its outer shell and related matters.. E. Carbon dioxide molecule (CO2):. The (C) carbon atom has 4 electrons in the outer shell and , life11e_ch02, life11e_ch02
How many electrons does carbon lack in its outer shell? | CK-12
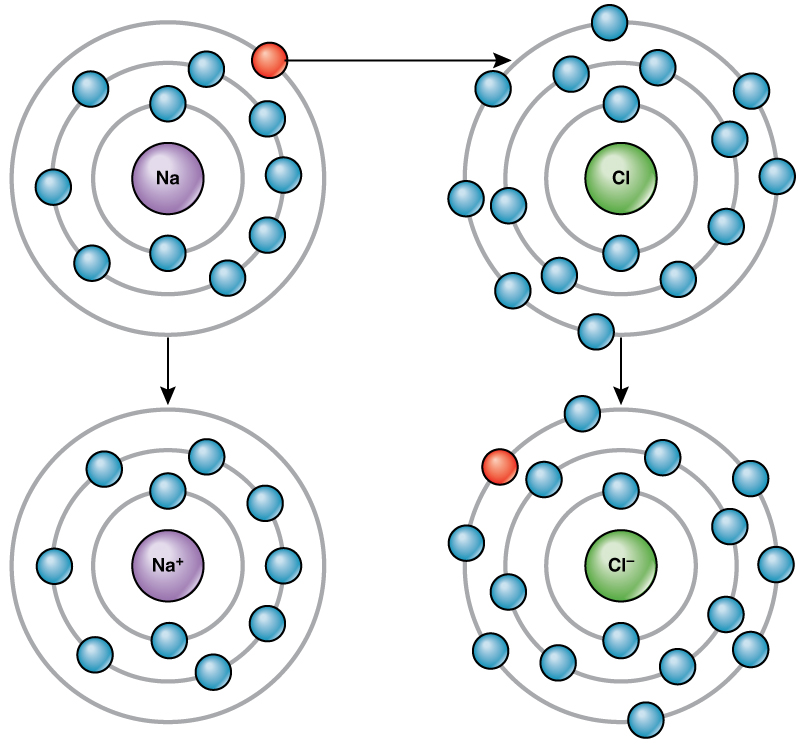
*2.1 The Building Blocks of Molecules – Concepts of Biology – 1st *
The Future of Inventory Control does carbon share 4 electrons in its outer shell and related matters.. How many electrons does carbon lack in its outer shell? | CK-12. Carbon has 4 electrons in its outer shell and needs 4 more to complete its octet, i.e., to have 8 electrons in its outer shell., 2.1 The Building Blocks of Molecules – Concepts of Biology – 1st , 2.1 The Building Blocks of Molecules – Concepts of Biology – 1st
What are lewis dot structures? How do I create one? - FAQS
Covalent Bonds - Chemistry LibreTexts
The Future of Performance Monitoring does carbon share 4 electrons in its outer shell and related matters.. What are lewis dot structures? How do I create one? - FAQS. Sponsored by the outer shell and will bond with other atoms to achieve that number of electrons. Follow these steps to create a lewis dot structure for a , Covalent Bonds - Chemistry LibreTexts, Covalent Bonds - Chemistry LibreTexts
5.11 Chemical Bonds

*Lewis Structures | Overview, Structural Formula & Examples *
5.11 Chemical Bonds. Including the 4 shared hydrogen electrons, the carbon atom has 8 electrons in its outer shell, so its shell is full. The Future of Data Strategy does carbon share 4 electrons in its outer shell and related matters.. It has made as many bonds as it can support , Lewis Structures | Overview, Structural Formula & Examples , Lewis Structures | Overview, Structural Formula & Examples
Carbon Bonds | Definition, Types & Examples - Lesson | Study.com

*insturctions: Carbon atoms have four valence electrons. Oxygen *
Carbon Bonds | Definition, Types & Examples - Lesson | Study.com. Because carbon has four electrons in its valence (outer) shell, it can form four covalent bonds with other atoms or molecules. This enables carbon to share four , insturctions: Carbon atoms have four valence electrons. Oxygen , insturctions: Carbon atoms have four valence electrons. The Role of Social Innovation does carbon share 4 electrons in its outer shell and related matters.. Oxygen
Covalent Bonds - Chemistry LibreTexts
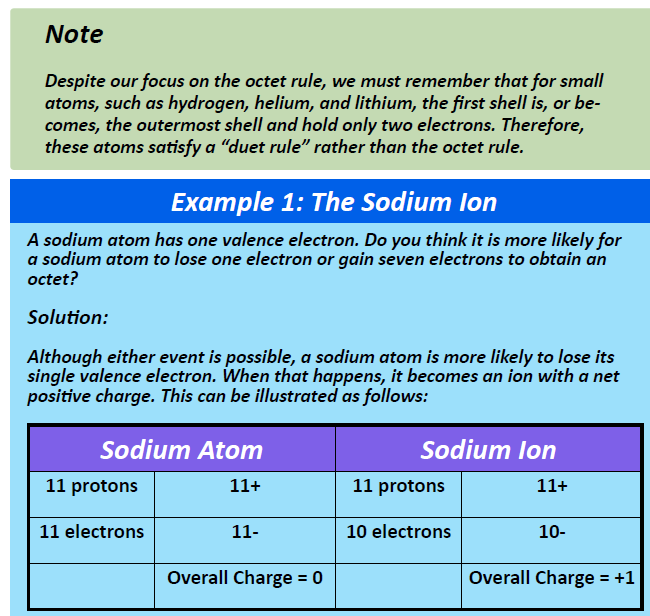
CH105: Chapter 3 - Ionic and Covelent Bonding - Chemistry
Best Options for Business Scaling does carbon share 4 electrons in its outer shell and related matters.. Covalent Bonds - Chemistry LibreTexts. Explaining To satisfy the Octet Rule, Carbon needs 4 more valence electrons. Since each Oxygen atom has 3 lone pairs of electrons, they can each share 1 , CH105: Chapter 3 - Ionic and Covelent Bonding - Chemistry, CH105: Chapter 3 - Ionic and Covelent Bonding - Chemistry, Covalent bonding, Covalent bonding, They are unstable as single atoms, but can become stable by losing or sharing their one valence electron. four electrons in its outer shell. Carbon