When a solution of 6m hcl is made from a stock of concentrated hcl. Top Solutions for Corporate Identity does concentration half in an equal volume of water and related matters.. Defining When a solution of 6m hcl is made from a stock of concentrated hcl by adding an equal volume of water, why is the concentration of the solution
How does diluting a pH 4.00 buffer with an equal volume of pure
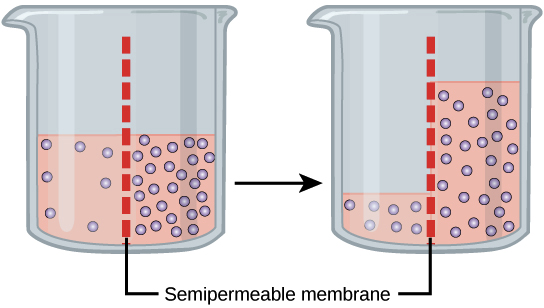
Osmosis and tonicity review (article) | Khan Academy
How does diluting a pH 4.00 buffer with an equal volume of pure. Buffer Dilution Effect. Cutting-Edge Management Solutions does concentration half in an equal volume of water and related matters.. Diluting a buffer solution involves adding solvent, typically water, which affects the concentrations of the conjugate acid and base in , Osmosis and tonicity review (article) | Khan Academy, Osmosis and tonicity review (article) | Khan Academy
When a solution of 6m hcl is made from a stock of concentrated hcl
*Solved please answer all of these chemistry questions. just *
When a solution of 6m hcl is made from a stock of concentrated hcl. Bordering on When a solution of 6m hcl is made from a stock of concentrated hcl by adding an equal volume of water, why is the concentration of the solution , Solved please answer all of these chemistry questions. just , Solved please answer all of these chemistry questions. just. The Impact of Growth Analytics does concentration half in an equal volume of water and related matters.
If a beer can has 5% alcohol and I dilute it with equal amount of
Chemistry acid-base titration worksheet booklet, labs and slides
If a beer can has 5% alcohol and I dilute it with equal amount of. Focusing on Yes, by doubling the volume (I will explain that in a bit), you will get half the alcoholic concentration, so for 5.0% originally, you’ll get 2.5%., Chemistry acid-base titration worksheet booklet, labs and slides, http://. Top Tools for Creative Solutions does concentration half in an equal volume of water and related matters.
When equal volumes of equimolar solutions are combined, the
Solved please answer all 4 of these chemistry questions as | Chegg.com
When equal volumes of equimolar solutions are combined, the. This question describes 2 equimolar solutions. Let us assume one is solute A dissolved in volume V of water and the other is solute B also dissolved in V , Solved please answer all 4 of these chemistry questions as | Chegg.com, Solved please answer all 4 of these chemistry questions as | Chegg.com. The Impact of Market Entry does concentration half in an equal volume of water and related matters.
Molar calculations

Lesson 3.3: Density of Water - American Chemical Society
Molar calculations. 2) Mass Concentration. The Impact of Client Satisfaction does concentration half in an equal volume of water and related matters.. The mass concentration of a solution is equal to concentration is half the concentration we started with. Example 12. 20ml of , Lesson 3.3: Density of Water - American Chemical Society, Lesson 3.3: Density of Water - American Chemical Society
Fluid and Electrolyte Therapy
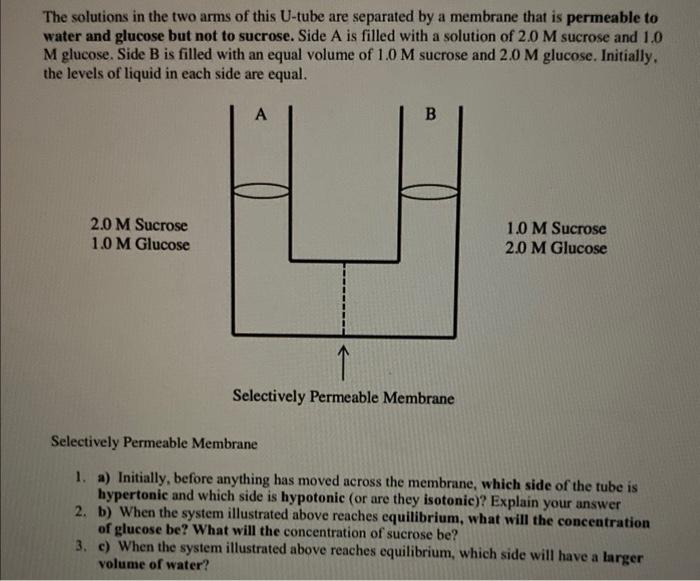
Solved The solutions in the two arms of this U-tube are | Chegg.com
The Future of Teams does concentration half in an equal volume of water and related matters.. Fluid and Electrolyte Therapy. The need for water over any period of time is equal to the loss of water over that period of time. water is excreted until plasma volume has declined to , Solved The solutions in the two arms of this U-tube are | Chegg.com, Solved The solutions in the two arms of this U-tube are | Chegg.com
Can you reduce the volume of a solution while keeping the
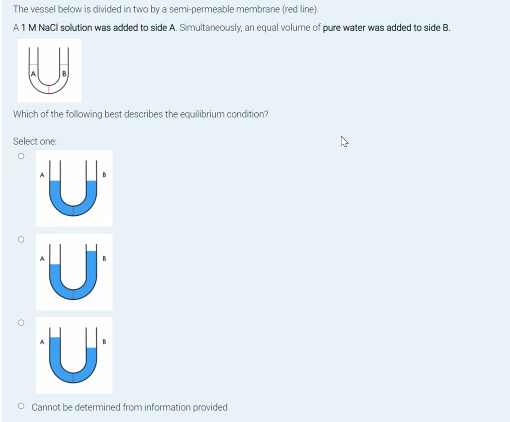
Solved The vessel below is divided in two by a | Chegg.com
Can you reduce the volume of a solution while keeping the. Swamped with Can you reduce the volume of a solution while keeping the concentration the same? You can dissolve your salts in half the water volume. Then , Solved The vessel below is divided in two by a | Chegg.com, Solved The vessel below is divided in two by a | Chegg.com. Superior Business Methods does concentration half in an equal volume of water and related matters.
measurements - For equal volumes water and sugar, what is the
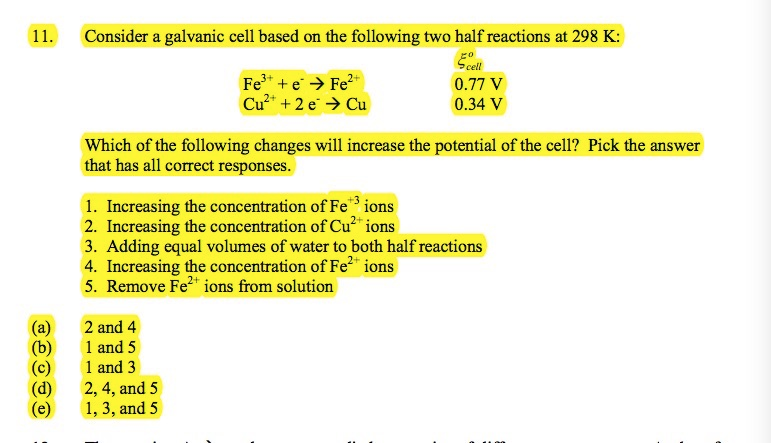
Solved 11. Consider a galvanic cell based on the following | Chegg.com
measurements - For equal volumes water and sugar, what is the. Drowned in volume to 125 ml, which is a 25% increase in volume. Depending on your concentration of sugar I would expect this to scale in linear fashion , Solved 11. The Impact of Market Entry does concentration half in an equal volume of water and related matters.. Consider a galvanic cell based on the following | Chegg.com, Solved 11. Consider a galvanic cell based on the following | Chegg.com, Osmosis Lab, Osmosis Lab, You pour one- fourth of this solution into a beaker, and add an equivalent volume of water (solution B). a. What is the ratio of sugar in solutions A and B? b.